ATS2015
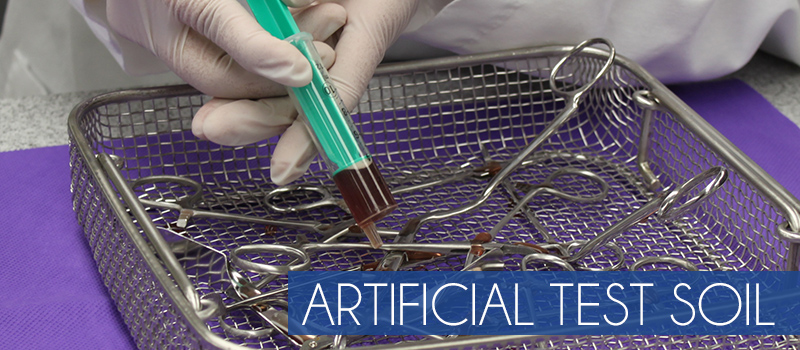
If you are a medical device manufacturer (MDM), 3rd Party Reprocessor or Independent Testing Lab that needs to comply with AAMI, FDA, CDC, CEN, Health Canada, CSA and other regulatory agencies, Healthmark ATS2015 is an easy and affordable answer. Formulated from extensive study of instruments soiled in real world situations, our Artificial Test Soil (ATS2015) contains the actual contaminants likely to remain on medical devices after clinical use. These include proteins (albumin), hemoglobin, carbohydrates, mucin, cellulose and lipids. Use of ATS2015 to soil your device, can help you validate your reprocessing instructions in compliance with regulatory requirements.
REAL
ATS2015 is comprised of the organic contaminants remaining on surgical instruments after clinical use, including protein (~33mg/ml, BCA assay), hemoglobin (~2.5mg/ml, TMB assay) and carbohydrates (~11mg/ml, Phenol-sulfuric acid assay), without the addition of sheep’s blood. These quantities are reflective of the “worst case” levels found to remain on instruments after patient use, but before reprocessing. The FDA and other international regulatory agencies specify that simulated-use testing should approximate as closely as possible the actual soiling the instrument is exposed to during clinical use. ATS2015 meets this requirement with extensive research (available upon request) to support it.
SIMPLE
Our standardized soil is ready-to-mix.
Medical device manufacturers must employ an organic challenge. ATS2015 is easy to use to determine and document the effectiveness of your cleaning protocol to remove organic materials from instruments or equipment.
VERSATILE
Healthmark ATS2015 can be applied to several applications, such as:
- Testing the cleaning instructions of medical devices
- Ensuring reprocessing adequacy and assurance
- Testing microbial load (bioburden) reduction
- Training personnel
ATS2015 can be inoculated with endotoxin, microorganisms, or other biologics to represent the residuals present on devices after patient use.
STABLE
Unlike the current organic challenge manufacturers use, ATS2015 offers less variability between batches. ATS2015 is described in various AAMI and ISO standards and FDA Guidance Documents and is well documented in peer-reviewed literature.
Our ATS provides long shelf life and is easily stored at room temperature until rehydrated for use.
COMPLETE
To provide a complete testing solution, ATS2015 can be used with other Healthmark Proformance™ products to validate the removal of organic soils based on the manufacturer’s cleaning protocol. Further, Proformance products provide a solution for manufacturers to provide their users with a recommended method to verify the cleaning process, as indicated by AAMI standards:
HEMOCHECK™
The HemoCheck blood test kit is an all-in-one test, providing easy to interpret results of blood residue to 0.1ug in just 30 seconds.
PROCHECK-II™
The ProCheck-II protein swab test kit is an all-in-one test, providing rapid results to measure residual protein on surfaces to 1.0ug.
CHANNELCHECK™
The ChannelCheck tests for blood, protein and carbohydrates simultaneously in virtually any lumened instrument, almost instantly.
TOSI®
The TOSI Instrument Washer Test is a surrogate device for stainless steel instruments that correlates directly to instruments soiled with blood during clinical use. It can be used for simulated-use testing to recommend cleaning protocols for such instruments as well as recommended for healthcare facilities as a method for verifying cleaning effectiveness.